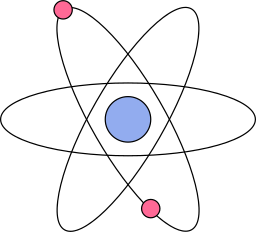
The Atom is hailed as the building block of the universe.
Atoms make up everything- the chair you're sitting on, the air you're breathing, the screen
you're reading from- and their discovery was a pivotal, revolutionary moment in the history of
physics, chemistry, science, and life as we know it.
But how fundamental is an atom? What are the building blocks of the building blocks?
Since the earliest proposal of the atom in ancient Greece, its structure and function have undergone
extensive modifications. While rudimentary, Niels Bohr's model of the atom, which resembles the
one on this page, is simple and well-known: a nucleus, composed of
positively charged protons and neutrally charged neutrons, with negatively charged electrons
orbiting around the nucleus in circular paths, much like planets around the sun.
But just as our model of the solar system evolved from an Earth-centric one to the
sun-centric one we know and are familiar with today, so did our model of the atom
and our understanding of the universe's most
fundamental particles.